For more information refer to ATAGIs advice on the Relative Timing of Administering Influenza and COVID-19 Vaccines in 2021. Rapid Diagnostic Testing for the Management of.

Why The 2021 Flu Vaccine Is So Important Queensland Health
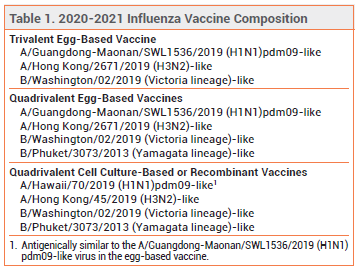
However the different compositions do not necessarily mean that there is a substantial difference in vaccine.
Influenza vaccine 2021 strain. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials. Influenza vaccines will be available in Australia from April 2021. UK health authorities find not a single case of influenza so far in 2021.
The egg-based technology for producing influenza vaccine was created in the 1950s. May increase miscarriage risk in younger women. United States Influenza Vaccines Distribution Demand 2010 - 2021 8.
The type with the greatest risk is highly pathogenic avian influenza HPAI. Effectiveness of Influenza Vaccines in the United States 2004 - 2021 9. The adjuvanted influenza vaccine Fluad Quad should not be co-administered with the adjuvanted subunit zoster vaccine Shingrix which is anticipated to be available in Australia from mid 2021.
Influenza Vaccine for 20212022 updates NACIs recommendations regarding the use of seasonal influenza vaccines. The recommendations for influenza vaccine composition for Fall 2021 were released in February 2021 by the World Health Organization. Bird flu is similar to swine flu dog flu horse flu and.
Golf legend Jack Nicklaus casts doubt on coronavirus death count. Further information for health professionals and consumers regarding influenza and COVID-19 vaccines is available from the Australian Technical Advisory Group on Immunisation ATAGI. Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds.
A key focus of NIAIDs influenza research program is developing a universal flu vaccine or a vaccine that provides robust long-lasting protection against multiple subtypes of flu rather than a select few. Such a vaccine would eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains potentially including. Those in phases later phases are urged to get their influenza vaccine as soon as they can and then have the COVID-19 vaccine.
CDN Newswire 28th June 2021 1909 GMT10. Seyhan Boyoglu-Barnum et al Quadrivalent influenza nanoparticle vaccines induce broad protection Nature 2021DOI. Flu shots are ineffective for seniors.
A DNA vaccine contains a small circular piece of DNA called a plasmid that includes genes that code for proteins of a flu virus. If an influenza vaccine has been inadvertently co-administered or given within a shorter interval than 14 days with a COVID-19 vaccine revaccination with either vaccine is not considered necessary. Global Vaccine for Influenza Market 2021 - Recent Trends Geographical Outlook Business Opportunities and Forecast to 2026.
From start to finishthe selection of which three strains to target with the vaccine to the production of the final productthe development process for the seasonal flu vaccine. When the vaccine is injected into the body cells read the. The safety of Quadrivalent Influenza Vaccine split virion inactivated was assessed in six clinical trials in which 3040 adults from 18 to 60 years of age 1392 elderly over 60 years of age and 429 children from 9 to 17 years of age received one dose of Quadrivalent Influenza Vaccine split virion inactivated and 884 children from 3 to 8 years of age received one or two doses of.
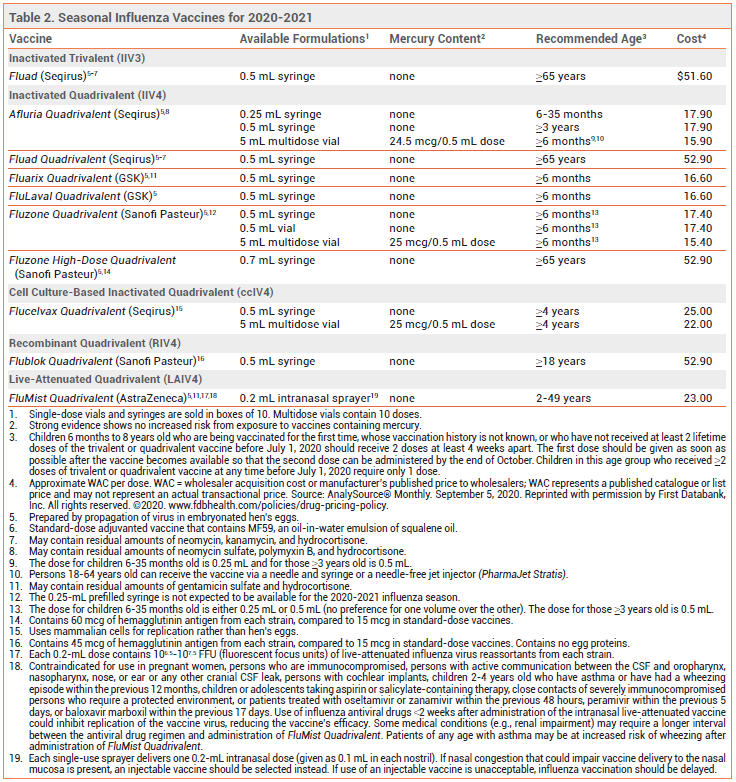
2 For the second year in a row the composition of the vaccine will depend on whether it is egg-based or cell-based or recombinant vaccine. The safety of concomitant administration of two adjuvanted vaccines has. Each season vaccine is generally designed to protect against three strains of influenza.
The vaccination program was rushed yet plagued by delays and public relations problems. Two A strains and one B strain. I1 New or Updated Information for 20212022 Guidance on the use of seasonal influenza vaccine in the presence of the novel coronavirus 2019 disease COVID-19 In light of the COVID-19 pandemic PHAC has developed additional guidance on seasonal influenza vaccination to.
MRInsightsbiz has recently published a report titled Global Vaccine for Influenza Market Growth 2021-2026 provides a high-quality and concise overview of the market taking into account information. 02082021 By Cassie B. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic.
The Australian Influenza Vaccine Committee AIVC recommendation for the composition of influenza vaccines for Australia in 2021 introduces two new strains to the NIP vaccines when compared to the composition of the vaccines for Australia in 2020. The 2021 vaccines will be quadrivalent influenza vaccines that contain the following four influenza strains the first two strains are new to the 2021 vaccine. Influenza vaccine production and distribution in the US are primarily private sector endeavors but during the 2020-2021 flu season as part of efforts to maximize flu vaccination by increasing availability of vaccine CDC purchased an additional 2 million doses of pediatric and 93 million doses of adult influenza vaccine to create a stockpile of vaccine in case of supply problems.
For the 2021-2022 flu season the Advisory Committee on Immunization Practices ACIP recommends annual influenza flu vaccination for everyone 6 months and older with any licensed influenza vaccine that is appropriate for the recipients age and health status including inactivated influenza vaccine IIV4 recombinant influenza vaccine RIV4 or live attenuated nasal spray influenza. Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the single. These months are needed because the process of producing a new vaccine involves many sequential steps and each of these steps requires a certain amount of time to complete.
A new A H1N1 like virus strain a new A H3N2 like virus strain. Coronavirus controls will only delay large outbreaks of endemic diseases like. It takes approximately five to six months for the first supplies of approved vaccine to become available once a new strain of influenza virus with pandemic potential is identified and isolated.
03052021 By Nolan Barton. 03102021 By Ramon Tomey.

A And B Strains Influvac 2021 2022 Inactivated Influenza Vaccine Abbott India Ltd Prescription Rs 900 Piece Id 22462865462
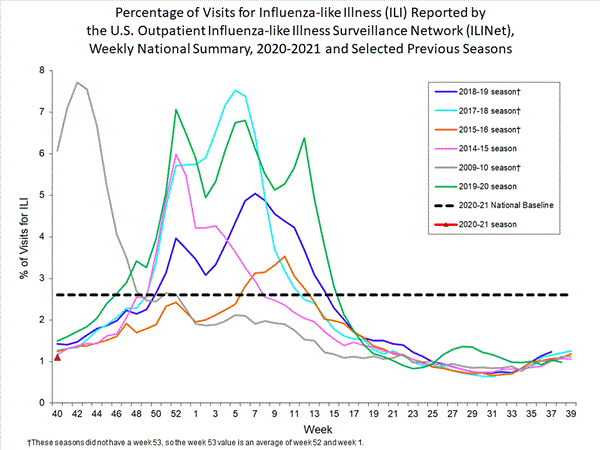
Fluview Summary Ending On October 3 2020 Cdc
Https Www Who Int Docs Default Source Wpro Documents Emergency Surveillance Seasonal Influenza Influenza 20210811 Pdf Sfvrsn B3fcfc77 69
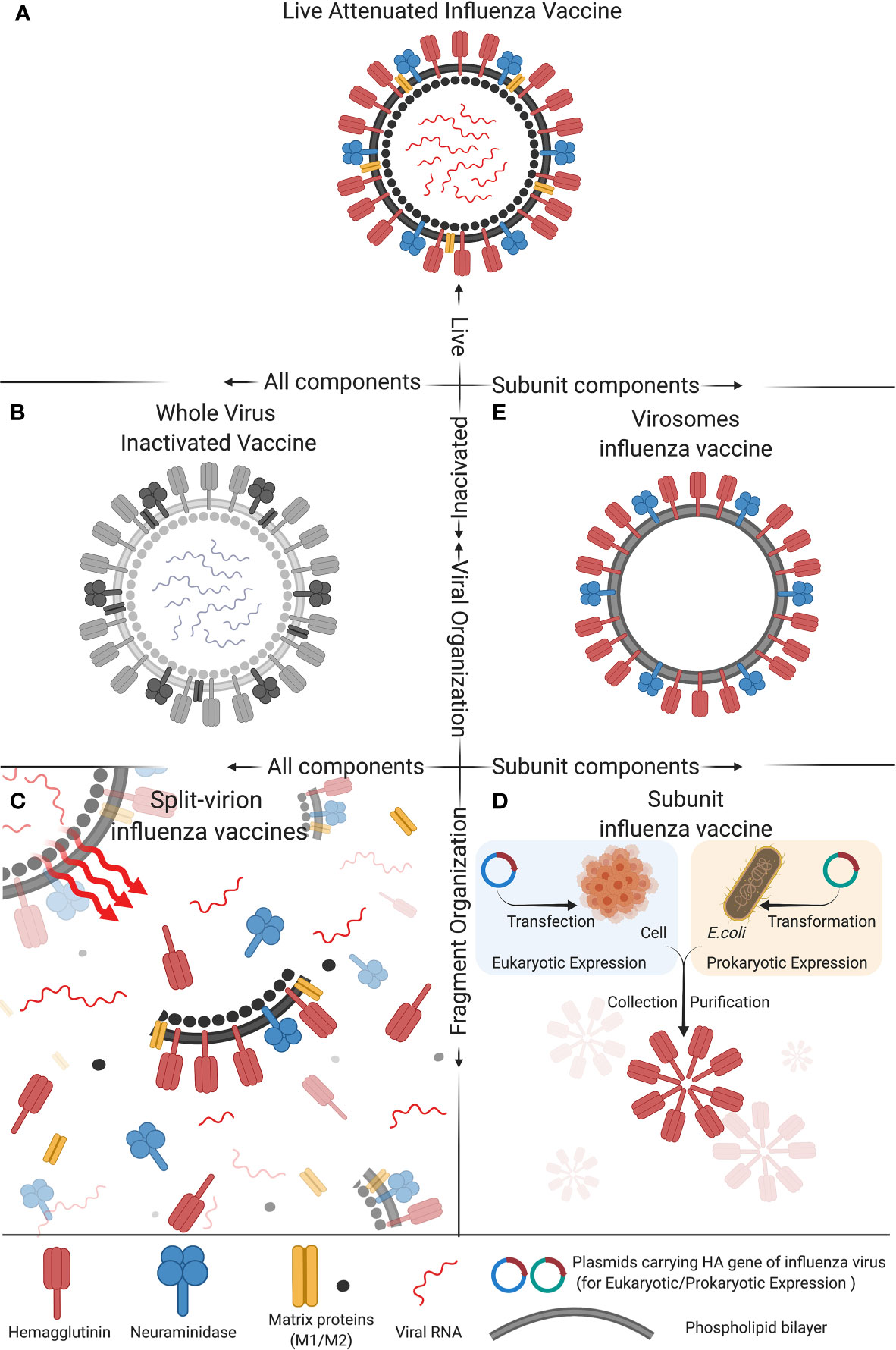
Frontiers Advances In Development And Application Of Influenza Vaccines Immunology

National Survey Attitudes About Influenza Pneumococcal Disease And Covid 19 National Foundation For Infectious Diseases
Southern Hemisphere 2021 Flu Vaccine Available International Medical Clinic Imc
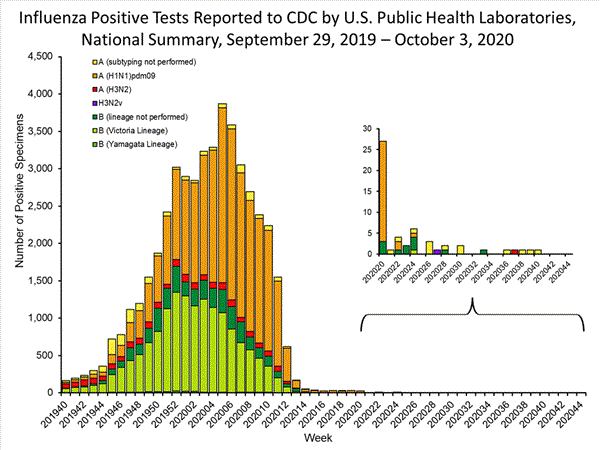
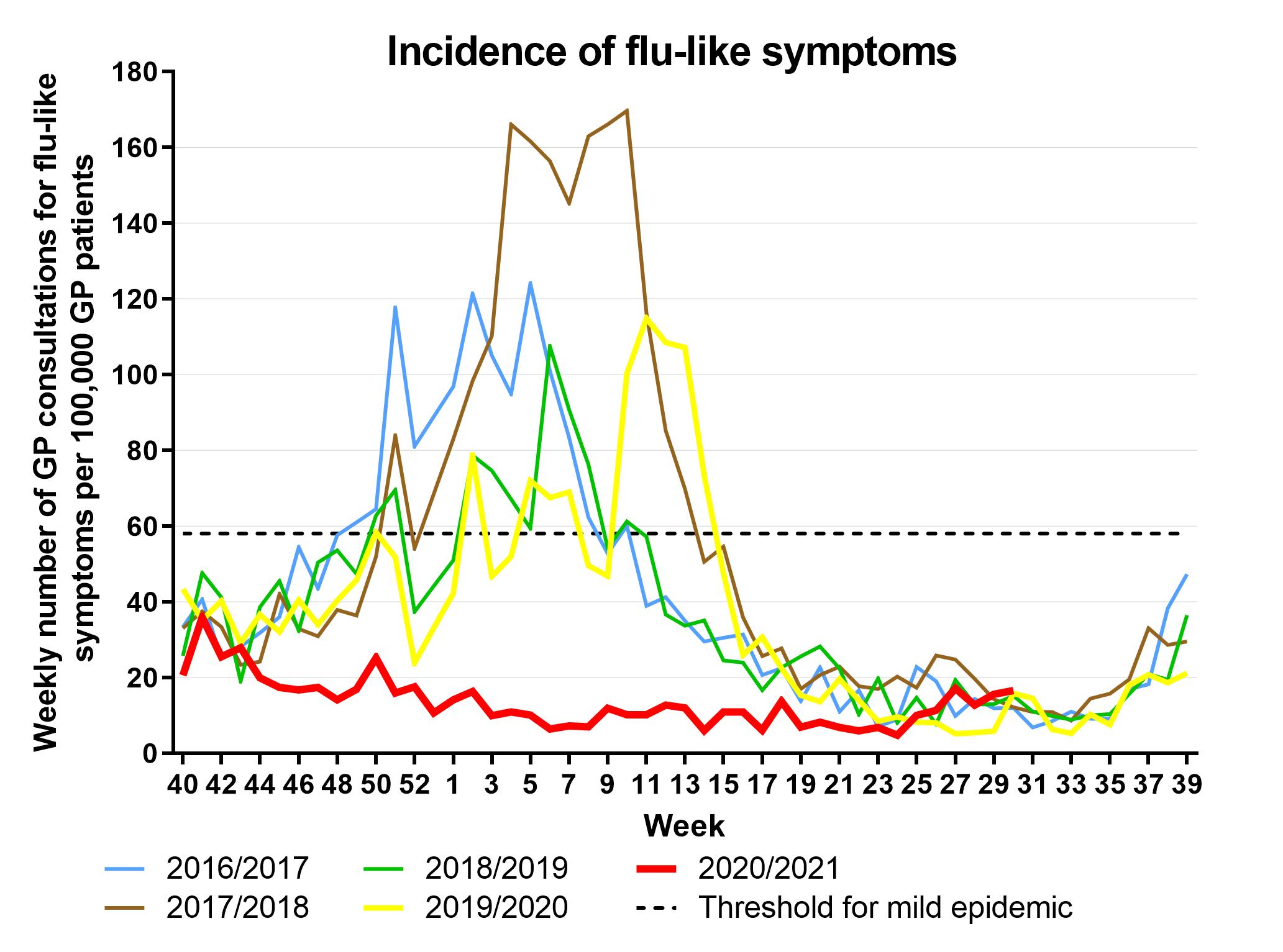
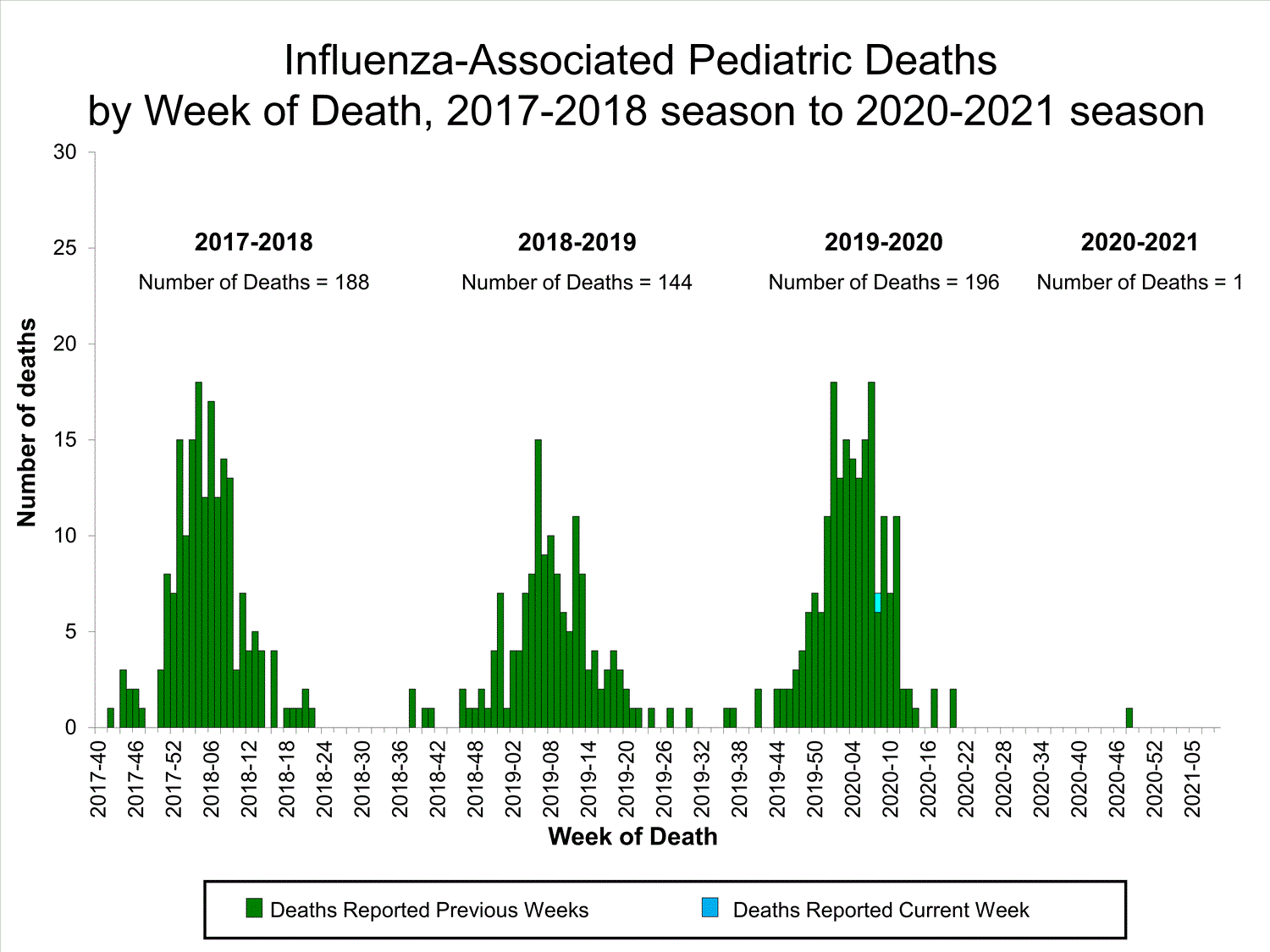
Fluview Summary Ending On October 3 2020 Cdc

Influenza Vaccine 2021 Be Well
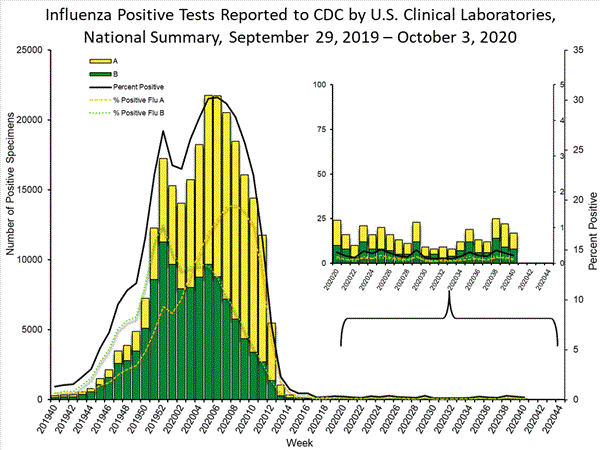
Fluview Summary Ending On October 3 2020 Cdc

2020 2021 Flu Shot Ingredients What Is In The Flu Shot And Why Fatherly
Influenza Vaccine For 2020 2021 The Medical Letter Inc

Recommended Composition Of Influenza Virus Vaccines For Use In The 2021 2022 Northern Hemisphere Influenza Season
Https Www Who Int Docs Default Source Wpro Documents Emergency Surveillance Seasonal Influenza Influenza 20210728 Pdf Sfvrsn 39dcc97a 74
Https Www Who Int Docs Default Source Wpro Documents Emergency Surveillance Seasonal Influenza Influenza 20210728 Pdf Sfvrsn 39dcc97a 74

Influenza Virus Characterisation Summary Europe February 2021

Avian Flu Diary Who Recommended 2021 Southern Hemisphere Flu Vaccine Composition Impact Of Covid 19 On Selection

Fluview Summary Ending On March 6 2021 Cdc